About the scan project
Photo credit: Vaincre la MucoviscidoseAbstract
Infections caused by multidrug-resistant Gram-negative bacteria result in significant mortality and morbidity worldwide. In line with this, all pathogens that received a ‘critical’ status by the recently established WHO priority list were drug-resistant Gram-negative species.
The reasons for limited success of pharmaceutical research programs in the area of antibiotics have been carefully analyzed: the main hurdle is the limited understanding how to get drugs into Gram-negative bacteria. Thus, there is a strong need for novel, innovative drugs against infections caused by Gram-negative pathogens. There is also a lack of tools to diagnose bacterial infections at deep body sites, e.g. on implant surfaces.
In the project SCAN (Siderophore Conjugates Against gram-Negatives), we apply a rational design approach to establish a targeting conjugate platform that can be used to both diagnose and treat bacterial infections (‘theranostics’ principle). The conjugates are actively transported into bacteria through their iron transport machinery that accepts siderophores as substrates. As this resembles the strategy of ancient Trojan warriors, the approach has been named the ‘Trojan Horse Strategy’. This concept has recently been validated clinically, a first drug (Fetroja) has been approved and is available to patients.
 Photo credit: Carsten Peukert
Photo credit: Carsten Peukert
 Photo credit: Carsten Peukert
Photo credit: Carsten Peukert
We will design and synthesize artificial siderophores that employ novel central scaffolds and combinations of iron-binding motifs. Those will be coupled with hitherto unexplored effectors: RNA polymerase inhibitors are employed as potent antibiotics, and chemiluminescent probes will be used for imaging. As a linkage between siderophore and antibiotic, cleavable, self-immolative linkers will be constructed. The conjugates will be characterized in cellular assays and in animal infection models. Their translocation and resistance mechanisms will be investigated by genetic and proteomic methods.
The project should yield novel antibiotic lead structures as well as activatable bacterial probes with proven efficacy in vivo to detect and treat infections. Taken together, the afforded antimicrobials and moreover the novel theranostics could be tools that allow for strain-specific, potent treatment and monitoring of bacterial infections, addressing a major medical need expressed by the WHO.
Background in the field
The overarching goal of the project SCAN (Siderophore Conjugates Against gram-Negatives) is to provide novel, innovative solutions for the diagnosis and the treatment of bacterial infections. These solutions are based on rationally designed small molecules that should enter the bacterial cell by an active transport mechanism, and that are conjugated with functional moieties to image and/or to kill the pathogens. The main objectives can be summarized in four aims that are defined and prioritized as follows:

 Photo credit: Vaincre la Mucoviscidose
Photo credit: Vaincre la Mucoviscidose
Objective & Rationale
The three teams from Germany, France and Israel aim to combine their expertise and previously obtained assets in chemical biology and imaging probes (Shabat), siderophore biology and chemistry (Schalk), and antibiotic drug conjugate discovery and development (Brönstrup), in order to rationally design next generation siderophore conjugates with hitherto unexplored functionalities.
As an overall outcome of the project, novel, innovative lead structures to diagnose and treat bacterial infections caused by medically important Gram-negative pathogens will be provided that fullfill a pre-defined product profile.
The following specific goals are pursued: Novel, chemiluminescent molecular imaging probes for the specific and sensitive detection of bacteria will be developed and characterized in cell culture, tissue and in small animals.
Targeting conjugates based on novel effector antibiotics and novel siderophore mimics against Gram-negative pathogens will be synthesized and characterized in cell culture and in small animals.
A profound understanding of the uptake and resistance mechanisms of conjugates prepared under 1 and 2 will be established.
Work Plan Synergy
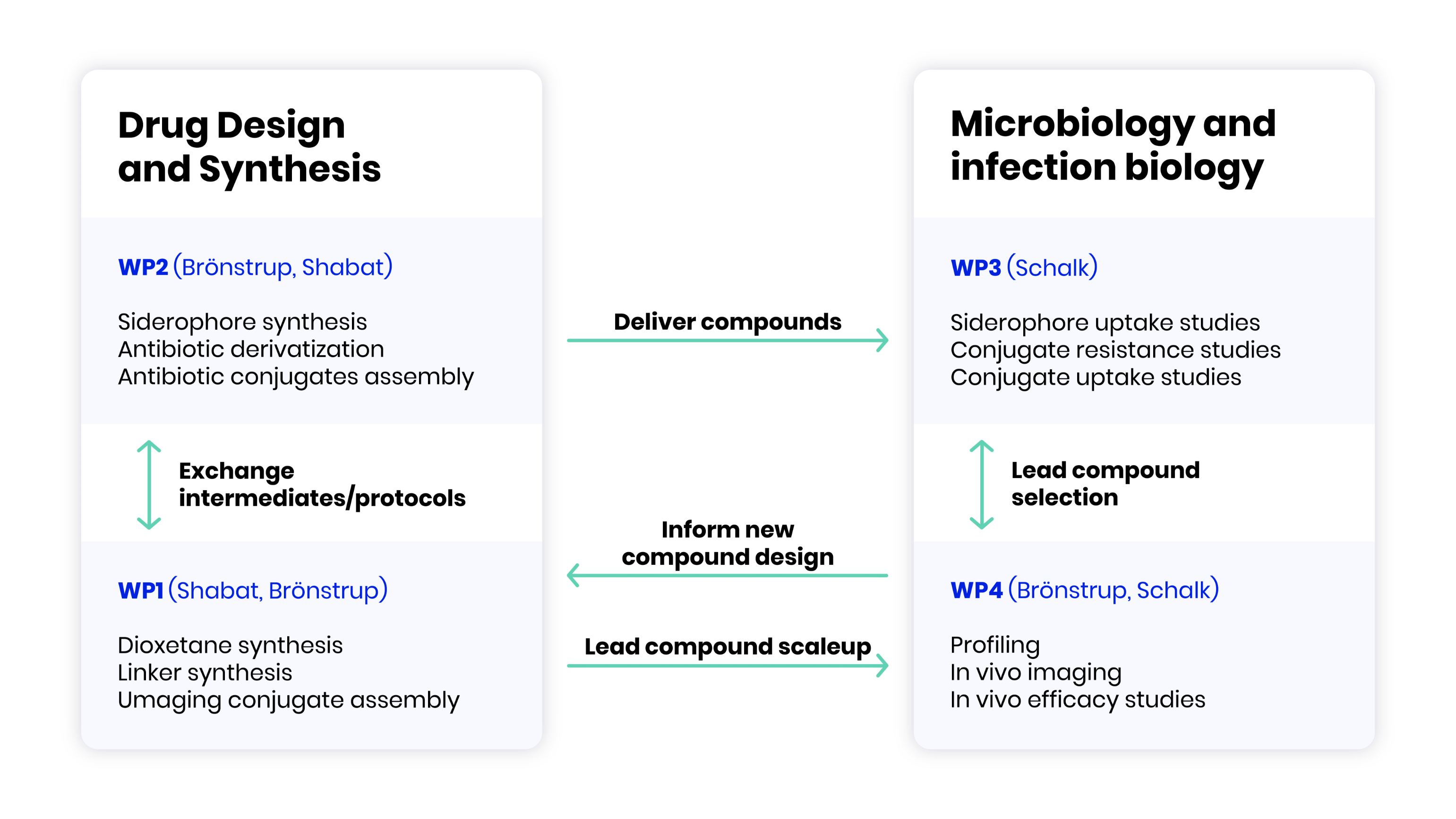
 Photo credit: Vaincre la Mucoviscidose
Photo credit: Vaincre la Mucoviscidose
Unmet medical and patient need
There is a broad consensus that antimicrobial resistance represents a serious threat for public health, because if the efficacy of antibiotics gets lost, there is no direct treatment for infections, but also other cornerstones of modern healthcare like safe surgery, immune compromising treatments or emergency medicine are in danger. The WHO has recently defined – for the first time in its history – a priority list of pathogens with a strong need for novel treatment options. Gram-negative pathogens fall into the top category, in particular carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa, and carbapenem/cephalosporin-resistant Enterobacteriaceae. The antibiotic resistence crisis exists globally, but pronounced local differences appear due different practices of antibiotic prescriptions and (mis)use.
Besides socioeconomic measures and improved treatment schemes with the currently available arsenal of drugs, there is an urgent need to discover and develop novel antibiotics that break bacterial resistance. However, the size and the level of innovation of the current antibiotic development pipeline is insufficient to adress this need. Multiple analysis come to the conclusion that an enhanced investment in early research and innovative therapeutic modalities against Gram-negative pathogens is warranted.
In addition, there is a strong need for an improved diagnosis of infections at deep body sites that are unaccesible for sampling (e.g. biomaterial-associated infections, endocarditis or brain infections). The problem can be adressed by non-invasive imaging methods, and the positron emission tomography (PET) agent 2-[18F]fluorodeoxyglucose (18F-FDG) is indeed in clinical use in western countries. However, FDG is unspecific and cannot differentiate between sterile inflammation and bacterial infections. Therefore, novel, specific imaging agents that selectively target bacterial pathogens are an area of active research.
 Photo credit: Vaincre la Mucoviscidose
Photo credit: Vaincre la Mucoviscidose
Funding
This project is funded by JPI-AMR and BMBF.